Imagine a world where matter doesn’t fit neatly into the categories of solid, liquid, or gas.
Scientists have just taken us a step closer to that reality.
Researchers have discovered an entirely new type of material, which they’re calling a “corralled supercooled liquid.”
What Makes This New State So Unique
In a normal liquid, atoms move around freely—think of them like people navigating through a crowded street.
They bump, shuffle, and jostle past one another constantly.
But researchers have found a way to “freeze” some atoms in place, forming a kind of atomic fence—or corral—that traps the remaining liquid atoms inside.
Once confined in these tiny atomic rings, the liquid behaves in ways scientists have never seen before.
It can remain fluid even when cooled far below its usual freezing point.
For instance, platinum can stay liquid at 350°C (662°F), which is more than 1,000°C colder than its standard freezing temperature.
Professor Andrei Khlobystov from the University of Nottingham, a co-author of the study, explains: “Our achievement may herald a new form of matter combining characteristics of solids and liquids in the same material.”
Why Understanding This Matters
With the exception of plasma, all natural states of matter are defined by how atoms and molecules move.
When a material transitions from liquid to solid, its atoms shift from freely moving to being locked into a rigid structure.
This transition is crucial for industries like metallurgy and pharmaceuticals because it affects how crystals form in solids.
However, because atoms in a liquid move so quickly, observing and understanding this transition has been extremely difficult—until now.
How Scientists Made the Discovery
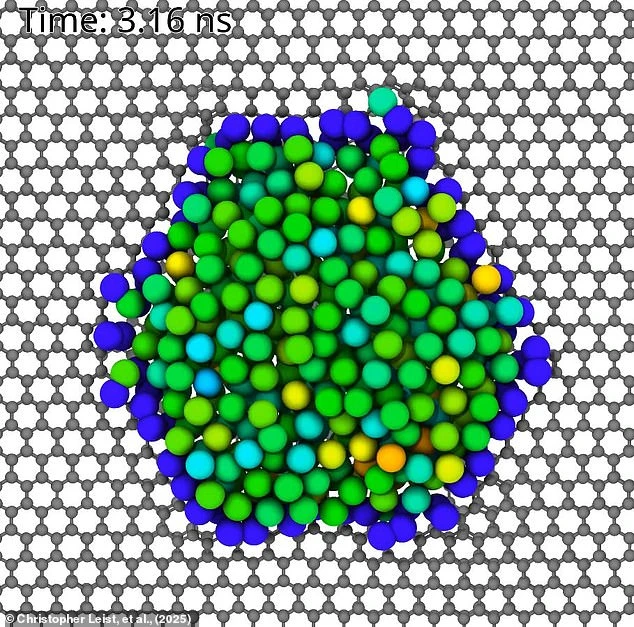
To explore this phenomenon, researchers turned to an electron scanning microscope to watch individual atoms in molten metal samples.
Dr. Christopher Leist from Ulm University, who performed these experiments, describes the process:
“We began by melting metal nanoparticles like platinum, gold, and palladium on a graphene support.
Graphene acted as a kind of stove to heat the particles, and as they melted, the atoms moved rapidly—just as expected.
But surprisingly, some atoms stayed perfectly still.”
The team realized these stationary atoms were getting stuck at atomic-scale defects on the graphene.
By using carefully targeted blasts of electrons, they could immobilize even more atoms.
Eventually, they formed complete rings of stationary atoms surrounding a tiny puddle of molten metal.
How Corralled Atoms Change Liquid Behavior
The stationary atoms had a profound effect on the metal’s solidification.
With only a few stationary atoms, crystals formed normally in the liquid and solidified.
But when the number of immobilized atoms was high, no crystals formed—the liquid remained trapped in a supercooled state.
This discovery enabled the creation of corralled supercooled liquids, a state of matter that continues to flow like a liquid hundreds of degrees below its freezing point.
And when these liquids eventually solidify, they form amorphous solids rather than the usual crystal structures, making them more similar to glass than to conventional metal.
Potential Industrial Impact
The implications are huge, particularly for rare metals like platinum.
Platinum is a key component in industrial catalysts, which speed up chemical reactions.
By creating new atomic arrangements, scientists may be able to improve catalysts, making them more efficient and longer-lasting.
Dr. Jesum Alves Fernandes of the University of Nottingham says: “This advancement may lead to the design of self-cleaning catalysts with improved activity and longevity.”
A Quick Look at the Natural States of Matter
For context, there are only four natural states of matter:
Solid: Atoms are tightly packed and held together by strong bonds. Solids keep their shape and volume.
Liquid: Atoms are close but not rigidly bonded, allowing them to flow and take the shape of their container.
Gas: Atoms move freely and independently, expanding to fill any space.
Plasma: When gas is energized, atoms split into ions and electrons that move independently. Plasma is affected by magnetic fields and makes up 99% of visible matter in the universe.
What’s Next
Researchers hope that experimenting with new corral shapes could unlock entirely new ways of using metals in technology and industry.
By controlling matter at the atomic level, we may be entering an era where metals behave in ways previously thought impossible.
Share on Facebook «||» Share on Twitter «||» Share on Reddit «||» Share on LinkedIn